How to check the FDA certificate of a non-contact thermometer?
What’s Infrared non-contact thermometer?
What’s the Con and Pro of the non-contact thermometer?
How to check the FDA certificate of a non-contact thermometer?

1.What’s Infrared non-contact thermometer?
Measuring a person’s temperature can be done in several ways. One method to measure a person’s surface temperature is with the use of non-contact infrared thermometers (NCITs). NCITs may be used to reduce cross-contamination risk and minimize the risk of spreading disease.
While typically 98.6°F (37.0°C) is considered a “normal” temperature, some studies have shown that “normal” body temperature can be within a wide range, from 97°F (36.1°C) to 99°F (37.2°C). Before NCITs are used, it is important to understand the benefits, limitations, and proper use of these thermometers. Improper use of NCITs may lead to inaccurate measurements of temperature.
2. What’s the Con and Pro of the non-contact thermometer?
Benefits
- Non-contact approach may reduce the risk of spreading disease between people being evaluated
- Easy to use
- Easy to clean and disinfect
- Measures temperature and displays a reading rapidly
- Provides ability to retake a temperature quickly
Limitations
How and where the NCIT is used may affect the measurement (for example, head covers, environment, positioning on forehead).
The close distance required to properly take a person’s temperature represents a risk of spreading disease between the person using the device and the person being evaluated.
3. How to check the FDA certificate of a non-contact thermometer?
How to check the FDA certificate of a non-contact thermometer?
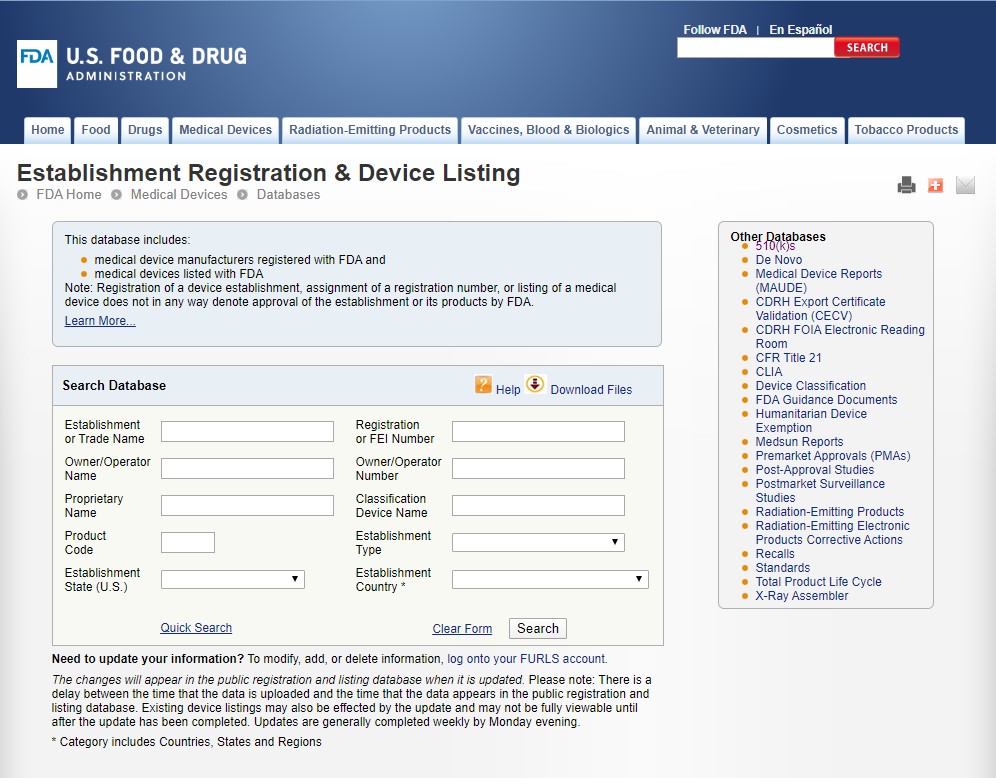
There are two database you can use for FDA information search, Establishment Registration & Device Listing and the 510(k) Premarket Notification.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
For Establishment Registration information, you can input the official English name of the company into the Establishment / Trade Name or Owner/Operator Name. Only one blank been filled would be enough.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm
After a company register in the FDA database, they need have






Recent Comments